High Performing, Animal Free Antibodies for Cardiac Assays
In vitro diagnostic (IVD) assays for detecting cardiac markers are essential to help treat or prevent cardiovascular disease (CVD). Successfully developing and manufacturing such products hinges on a reliable long-term supply of essential raw materials – namely, cardiac marker antibodies and antigens. Medix Biochemica Group is your partner of choice for high quality IVD materials and is a global, market-leading supplier to the IVD industry.
Animal free in vitro manufacturing of antibodies
- We manufacture antibodies in vitro in cell culture
- No serum or other animal derived components are used during manufacturing
- Our antibodies are produced in European Union
Excellent performance and high sensitivity in cardiac assay
- Our antibodies have been tested extensively during R&D and manufacturing
- We offer matched antibody pair recommendations
Cardiac Troponin I (cTnI)
Cardiac Troponin I is a major component of the ITC complex (comprising troponins I, T, and C), which has an essential role in regulating contraction in skeletal and cardiac muscles. The presence of cTnI in serum is often used to help diagnose myocardial infarction.
Our cTnI antibodies recognize a range of different epitopes, including the N-terminal region 39-52, which is widely regarded as the “gold standard” epitope for cTnI detection. These are complemented by both native and recombinant cTnI antigens, which include the intact ITC complex.
| Monoclonal antibody | Catalogue number | Epitope | Recommended pairs |
| RC9750 | 700050 | N-terminal region 39-52 | 9701 / RC9701, 9705 |
| 9707 and RC9707 (recombinant) | 100180 / 140020 | C-terminal region 190-195 | RC9750 |
| 9701 and RC9701 (recombinant) | 100129 / 140000 | Mid-region 85-95 | RC9750 |
| 9705 | 100125 | N-terminal region 21-30 | RC9750 |
| Antigen | Catalogue number |
| Cardiac Troponin I native antigen | 550-11 |
| Cardiac Troponin ITC native antigen | 550-08 |
| Cardiac Troponin I recombinant antigen | 610102 |
Medix Biochemica RC9750 overperforms market-leading competitor mAb in cTnI assays
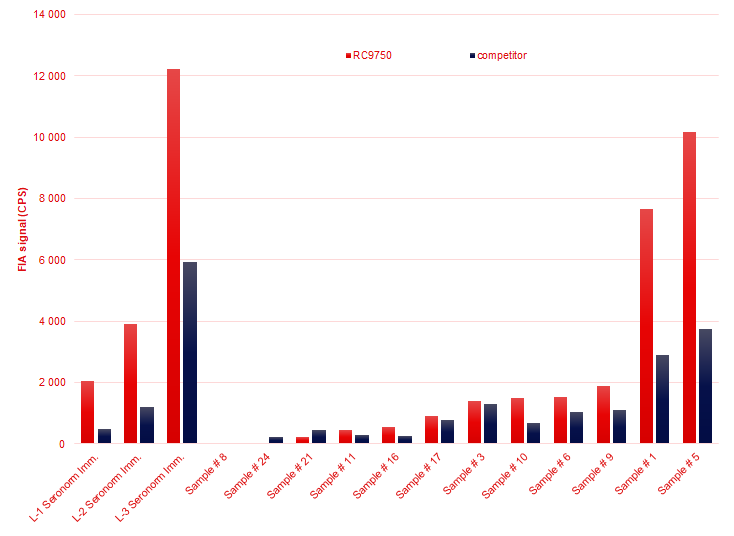
RC9750 is an excellent capture mAb for epitope region of 39-52.
N-terminal prohormone of brain natriuretic peptide (NT-proBNP)
NT-proBNP is a prohormone that is cleaved to release the brain natriuretic peptide (BNP). Both NT-proBNP and BNP show increased plasma concentrations in patients with heart failure.
We offer a selection of antibodies targeting different regions of the NT-proBNP protein, including antibodies to the N- and C-termini, as well as high quality NT-proBNP and BNP antigens.
| Monoclonal antibody | Catalogue number | Epitope | Recommended pairs |
| 1309 | 100710 | C-terminal region 59-70 | 1306 / RC1306, 1308 |
| 1306 and RC1306 (recombinant) | 100521 / 140010 | N-terminal region 12-28 | 1309, 1312 |
| 1308 | 100712 | N-terminal region 22-34 | 1309, 1312 |
| 1312 | 100717 | C-terminal region 59-70 | 1306 / RC1306, 1308 |
| Antigen | Catalogue number |
| NT-proBNP recombinant antigen | 610090 |
| Brain Natriuretic Peptide-32 (BNP-32), Synthetic | 129-10 |
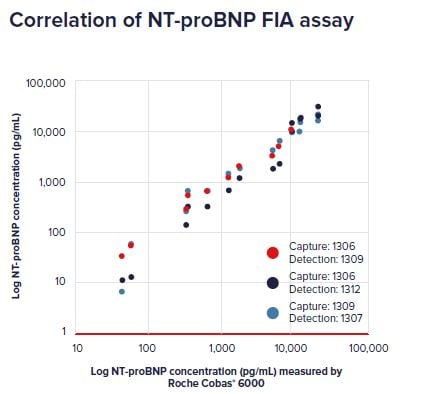
Correlation NT-proBNP concentrations in clinical samples between reference IVD method (Roche Cobas® 6000) and a fluoroimmunoassay (FIA) using NT-proBNP antibodies 1306 or 1309 for capture, and 1307, 1309 or 1312 for detection.
NT-proBNP antibodies demonstrate excellent assay sensitivity correlating well with the reference IVD assay.
D-dimer
D-dimer is a degradation product of crosslinked fibrin, used to diagnose pulmonary embolism and deep vein thrombosis (DVT). Our D-dimer antibodies have been tested for fluorescence immunoassay (FIA) and immunoturbidimetry (IT) to identify the best performing antibody pairs and are accompanied by a selection of native D-dimer antigens.
| Monoclonal antibody | Catalogue number | Application | Recommended pairs |
| 1408 | 100799 | FIA | 1409 |
| 1409 | 100800 | FIA | 1408 |
| 1401 | 100204 | FIA | 1408, 1409 |
| 1403 | 100228 | IT | 1404 |
| 1404 | 100479 | IT | 1407 |
- Best antibody pairs for FIA: 1409-1408, 1408-1409, 1401-1409, 1401-1408
- Best antibody pair for IT: 1403-1404
| Antigen | Catalogue number |
| Native D-Dimer | 200-09, 200-12, 200-13 |
Supporting IVD assay development and manufacturing
Identifying suitable antibodies – especially matched antibody pairs - can be one of the most time-consuming steps of IVD assay development. We expedite this process by validating our antibodies for pairwise use across multiple applications and can supply custom formulations where necessary to help streamline your workflow. Additionally, we offer a comprehensive selection of antigens for use as controls or reference standards.
To discuss your needs with one of our team, please get in touch today email us at sales@medixbiochemica.com






