Looking Ahead: The Future of IVD Cancer Testing
The first documented case of human cancer was 2 700 years ago, but almost 3 000 years later we continue to be plagued by this devastating illness.1 Cancer is now the no. 2 killer in the world and the global medical and scientific community is devoting time, thought and cutting-edge expertise to improving the prevention, diagnosis and treatment of this deadly disease.2 What role does in vitro diagnostic (IVD) testing currently play in the global battle against this disease, and what will its role be in future?
Cancer in Our Society
In 2018 cancer was responsible for 9.6 million deaths across the world (one in every six deaths).3 That’s more than AIDS, malaria and tuberculosis combined. It’s second only to heart disease as the leading cause of death, with both incidence and mortality growing each year.2,4 Without significant progress in the field of oncology prevention and treatment, the number of annual cancer deaths could reach 13.2 million by 2030.4
To give some perspective on the enormity of this health burden, consider that a total of 6 881 401 global COVID-19 deaths had been reported by mid-May 2023.5 It certainly is sobering to consider that cancer kills more people in one year than COVID-19 has since the start of the pandemic.
If robust prevention, screening and early-detection measures are in place – and are followed by access to quality-assured diagnostics and prompt and effective treatment and care – a significant number of these deaths can and should be avoided.2
The Current Role of In Vitro Diagnostics (IVDs) in the Screening, Diagnosis and Treatment of Cancer
Major advances in technology and immunochemistry have led to the development of accurate and inexpensive IVD tests, including point-of-care (POC) testing and self-testing.2 The growing inclusion of cancer IVD tests in the second WHO Model List of Essential In Vitro Diagnostics for use in clinical laboratories (WHO EDL), illustrates their increasingly important role in cancer prevention, diagnosis, treatment and care.2
IVD tests play a vital role throughout the cancer care pathway and therefore shouldn’t be described in isolation.2,6
IVDs can be vital for the early detection of cancer2
Between 30% and 50% of human cancers are preventable, and several can be screened for using IVD tests.2 Generally, cancers are easier to treat when they’re detected early, resulting in better patient outcomes as well as a likely reduction in the cost of treatment, with substantial savings to health systems.2
Cancer-specific IVD screening tests
IVD tests specifically used for cancer screening make use of diagnostic pathology techniques such as cytology, surgical pathology, flow cytometry and molecular testing.2 Additionally, fluid biomarkers in blood, urine, stool or other bodily fluids can be used to screen for cancer.2 These biomarkers are proteins or other substances that cancer cells make in higher amounts than normal cells do.
Two examples of tests which screen for biomarkers are:
- Prostate-specific antigen testing for prostate cancer screening2
- Faecal immunochemical test (FIT), which can be used to screen for colorectal cancer.2 FIT detects human haemoglobin, which could indicate bleeding in the gastrointestinal tract2
Predicting cancer risk
IVD testing can answer important questions about a patient’s health status, including their risk or predisposition for developing a certain cancer.2
Guiding post-diagnosis clinical decisions
IVD tests also help clinicians to understand the stage of disease so that they can make appropriate treatment decisions following diagnosis.2
Increasingly, genomic markers such as tumor gene mutations, patterns of tumor gene expression and nongenetic changes in tumor DNA are also being used as tumor markers – either as part of the diagnostic pathology or as part of blood or other fluid testing.2
Determining the appropriate therapy
IVD tests are rapidly becoming an integral part of understanding what therapy may be best for a cancer patient.2 Companion diagnostics highlighting the nature and expression of a patient’s tumor, often determined using IVD techniques, assist clinicians to understand the likelihood of a specific patient benefitting from a particular treatment.2
For example:2
- The presence of a BCR-ABL fusion gene can confirm whether the use of a drug like imatinib is likely to be successful in the treatment of a patient with chronic myeloid leukaemia (CML)
- Oestrogen receptor testing can help determine the likelihood of a breast cancer patient benefitting from tamoxifen
- PD-L1 and/or microsatellite instability testing can assess the likelihood of a patient benefitting from pembrolizumab, whilst BRAF testing of tumor cells helps to predict a patient’s likely response to BRAF inhibitors
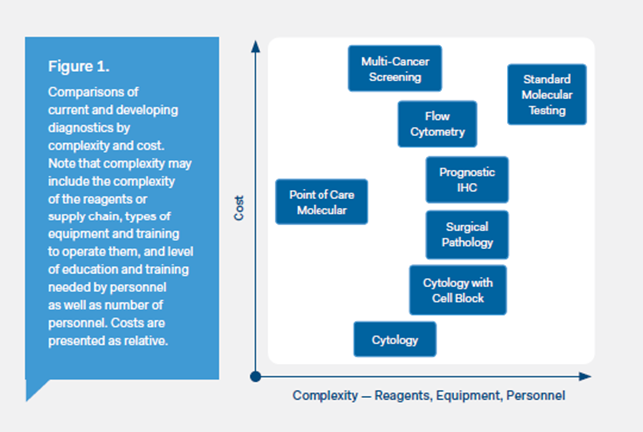
Figure 1: Snapshot of IVD tests currently available within the field of oncology
Source: The role of in vitro diagnostics in early detection and treatment of cancer (American Society for Clinical Pathology, et al. 2021)2
Enjoying this Article? Why not subscribe to our newsletter to have them delivered straight to you inbox.
IVD Testing in Cancer: Trending Techniques and Technologies
Trends in cancer detection
Investment in cancer detection through IVD testing has grown rapidly in recent months.7
A 2022 GlobalData report confirms that investment towards and growth in minimally invasive and high-performing oncology tests will continue to drive scientific innovation and the IVD market for years to come.7
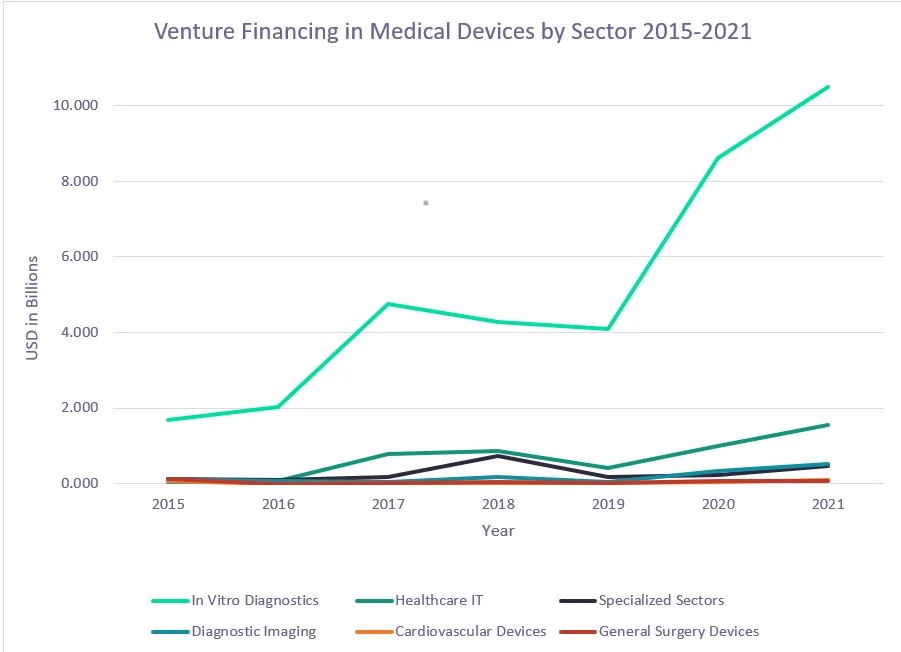
Source: Cancer detection drives in-vitro diagnostics venture financing deals
(GlobalData. July 21, 2022)7
Trends in precision medicine
Early detection through techniques such as IVD testing is important. However, precision medicine – quantitation, multiplexing and extremely precise identification of markers – has become the new goal in cancer testing.4 Tools once thought only to be useful in a research setting are now being adopted in clinical practice.4 Among the most promising platforms are digital PCR, mass spectrometry, chromosome analysis, tissue microarrays, next-generation sequencing, and cell signaling pathway testing.4 Knowing the precise nature of what has been detected by IVD tests is essential to ensuring a good outcome for the patient.
Medix Biochemica Offers Expertise, Quality and Customization in Supporting IVD Testing Development
Medix Biochemica offers a variety of high-quality products that play an important part in IVD oncology test development and manufacturing, including materials used for:6
- PSA (prostate-specific antigen) biomarker testing, used in prostate cancer testing
- CEA (carcinoembryonic antigen) tumor markers, for detecting multiple tumors and to track treatment effectiveness
- Pepsinogen 1 and 2, for detection of gastric cancers
- CD138, for myeloma detection by immunohistochemistry
- Tumor mutational profiling by genetic testing
What sets Medix Biochemica apart in this rapidly growing field?6
- At Medix Biochemica we are focused on quality, we ensure rigorous quality control and testing in development, and we test every lot that is manufactured. This allows us to provide our clients with extensive data on the products they choose.
- Our production is in vitro and extremely scaleable, meaning we can produce the substantial quantities that large-scale IVD companies may need.
- We work closely keep to market trends, ensuring that we’re developing products of the highest quality and with real clinical relevance.
Due to our in-house expertise and sophisticated techniques, we can develop specific antibodies, antigens, and DNA polymerases to meet our customers’ unique needs. So, if a product is not yet in our catalog, we’ll work with you to develop the customized, specialized solutions your product requires.
To stay up to date on advances in the IVD testing space in oncology and across all healthcare settings, subscribe to our newsletter .
References:
- Faguet GB. A brief history of cancer: Age-old milestones underlying our current knowledge database: A brief history of cancer. Int J Cancer. 2015;136(9):2022-2036. doi:10.1002/ijc.29134.
- American Society for Clinical Pathology, Foundation for Innovative New Diagnostics, Union for International Cancer Control. The role of in vitro diagnostics in early detection and treatment of cancer. 2021.
- Cancer. World Health Organization. Accessed May 24, 2023. https://www.who.int/health-topics/cancer#tab=tab_1.
- 6 promising IVD technologies that will change cancer testing. Medical Device and Diagnostic Industry. Accessed May 24, 2023. https://www.mddionline.com/news/6-promising-ivd-technologies-will-change-cancer-testing.
- Coronavirus death toll. Worldometer. Accessed May 23, 2023. https://www.worldometers.info/coronavirus/coronavirus-death-toll/.
- Expert opinion. Interview with Maiju Palokangas, Sr. Product Manager, Immunodiagnostic reagents, Medix Biochemica, and Laurence Ringenbach), Medix Biochemica. 03 May 2023.
- Cancer detection drives in vitro diagnostics venture financing deals. Medical Device Network. Accessed May 24, 2023. https://www.medicaldevice-network.com/comment/cancer-diagnostics-venture-financing-deals/.
- Beltrán-García J, Osca-Verdegal R, Mena-Mollá S, et al. Epigenetic IVD tests for personalized precision medicine in cancer. Front Genet. 2019;10:621. doi:10.3389/fgene.2019.00621.




