Immunotherapy, such as CAR T-cell treatment, is widely recognized as the fourth pillar of cancer treatment, alongside traditional treatment methods like surgery, radiation therapy and chemotherapy.1 Let’s look at CAR T-cell therapy in more detail and discuss how it’s enhanced by the use of specialty reagents.
CAR T-cell treatment and achieving specificity
CAR T-cell treatment is a type of cancer immunotherapy. Disease-fighting T-cells (white blood cells) are collected from the patient and genetically modified to become chimeric antigen receptor (CAR) T-cells, which recognize the patient’s cancer cells. These specially modified T-cells are grown and multiplied in a laboratory, and then infused back into the patient’s bloodstream to target and attack the cancer cells.2
The diagram below from the National Cancer Institute illustrates the CAR T immunotherapy process.3
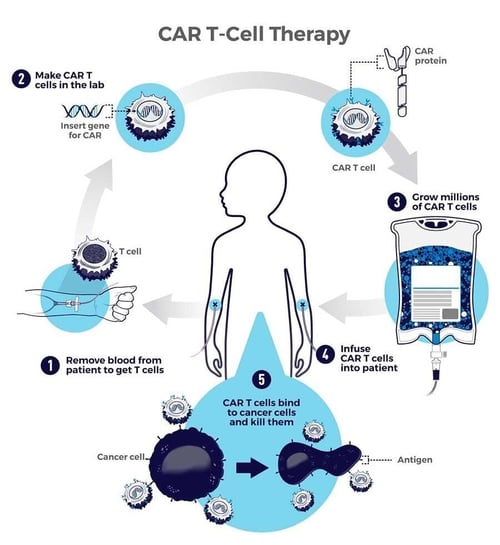
Figure 1: Steps in CAR T-cell therapy process - National Cancer Institute, 20133
CAR T-cell therapy is a type of treatment in which a patient's T cells are genetically engineered in the laboratory so they will bind to specific proteins (antigens) on cancer cells and kill them. (1) A patient's T cells are removed from their blood. Then, (2) the gene for a special receptor called a chimeric antigen receptor (CAR) is inserted into the T cells in the laboratory. The gene encodes the engineered CAR protein that is expressed on the surface of the patient's T cells, creating a CAR T cell. (3) Millions of CAR T cells are grown in the laboratory. (4) They are then given to the patient by intravenous infusion. (5) The CAR T cells bind to antigens on the cancer cells and kill them.3
Click here to read more about the benefits and challenges of CAR T-cell therapy
The role of single chain fragment variables in CAR T-cell therapy
The antigen-binding counterpart of a CAR is an antibody fragment known as a single chain variable fragment (scFv).4
“The single-chain fragment variable (scFv) that contains the complete antigen-binding domains of a whole antibody, has several advantages such as a high specificity and affinity for antigens, a low immunogenicity, and the proven ability to penetrate tumor tissues and diffuse.”
- Muñoz-López P, 2022, Unit of Investigative Research on Hemato-Oncological Diseases, Hospital Infantil de México Federico Gómez, Mexico City, Mexico4 |
scFv recognizes a membrane protein associated with a cancer cell and, because it has a high specificity for antigens, it can ‘arm’ the CAR T-cells to target and attack the cancer cells.4,5 This is the main format used in CAR T-cell therapy.6
Single-domain antibodies and their advantages
Conventional antibodies have a Y-shaped structure and typically consist of two ‘heavy’ and two ‘light’ protein chains.7 A single-domain antibody (sdAb or nanobody), however, is a heavy-chain-only antibody that has one ‘domain’ or functional part, termed VHH (variable domain of heavy-chain), that is responsible for antigen recognition.8
The sdAbs used in CAR T-cell therapy are produced by and sourced from llamas and other camelids, including camels and dromedaries.8
Due to their high stability, small size and modular shape, there is a growing trend in CAR T-cell treatment toward using sdAbs.
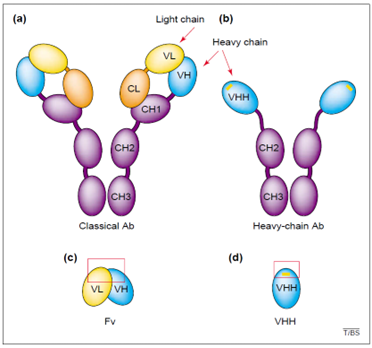
Figure 2: Fragments of classical vs. heavy-chain antibodies - TRENDS in Biochemical Sciences, 20019
Schematic representation of (a) a classical antibody, (b) a heavy-chain antibody of camelids, and (c and d) their respective antigen-binding fragments. The antigen-binding site of the VH–VL pair or of the VHH is denoted by the red boxes. The yellow bars in b and d indicate a frequently occurring inter-loop disulphide bond within the VHH.
Abbreviations: Ab, antibody; CH1–3, first, second and third constant heavy-chain domains; CL, constant light-chain domain; Fv, variable fragment; VH, variable domain of heavy chain; VHH, variable domain of heavy-chain antibody; VL, variable domain of light chain.9
“The camelid VHH domain, from heavy-chain-only antibodies, is a versatile tool for life science applications. Bolstered by its small size, modular shape, and diverse set of CDR interactions against protein and hapten targets, VHH domains provide opportunities not possible using conventional antibodies. VHH modularity provides opportunities for building blocks in antibody therapies, such as multi-specifics and CAR T cell therapy, as well as agile conformationally selective binding agents. The development and increasing use of synthetic VHH libraries, with userdefined diversity, along with the ability to introduce molecular switches, such as pH control, will allow VHH domains to play a pivotal role as functional ‘add-ons’ to create next-generation therapeutics and affinity reagents.”
- Hoey JR, 2019, Department of Chemistry and Biochemistry, Northern Illinois University, DeKalb, USA8 |
scFvs vs sdAbs: A summary
scFvs are more traditionally used in CAR T-cell treatment, but sdAbs are now being used more frequently. This table summarizes the differences between the two:6
| |
scFvs |
sdAbs |
| Structure |
Composed of the variable regions of the heavy (VH) and light (VL) chains of an antibody, connected by a flexible peptide linker, about 25 kDa in size |
Derived from camelid heavy-chain-only antibodies, consisting of a single monomeric variable antibody domain (VHH), about 12–15 kDa in size |
| Usage |
Traditionally used in CAR T-cell therapies as the antigen-recognition domain, derived from conventional antibodies |
Increasingly used in CAR T-cell therapies because of their small size, high stability and unique epitope binding |
| General advantages |
- Retain the antigen-binding specificity of full-length antibodies
- Smaller than full-length antibodies
- Easy to produce
- Effective in targeting cancer cells
|
- Better tissue penetration
- Reduced immunogenicity
- High specificity and affinity, making them effective in targeting cancer cells
|
Technical know-how at Diaclone
Diaclone, part of the Medix Biochemica group, specializes in scFv and sdAbs for use as raw materials in CAR T-cell therapies and assays.6
Diaclone is able to generate scFv and VHH against their target, using phage display technology which permits the selection of candidates as scFv or VHH formats (identical formats to those used in CAR T-cell therapy).6
Case Study: Development of VHH Anti-murine IFNg: First llama project success at Diaclone
While CAR T immunotherapy is the treatment process of targeting cancer via the infusion of genetically engineered T-cells, a CAR T-cell assay is a test used to assess the function, quality and effectiveness of CAR T-cells in a laboratory setting, either before or after the therapy is administered.6
Examples of CAR T-cell assays include:6
- Cytotoxicity assays: Measuring the ability of CAR T-cells to kill the targeted cancer cells
- Cytokine release assays: Measuring the release of specific signaling molecules by CAR T-cells, indicating their activity
- Proliferation assays: Assessing how well CAR T-cells divide and expand in the patient’s body
When using these specialty reagents to enhance CAR T-cell therapies and assays, it's important to remember that they’re only one part of a complex system. A particular scFv might work in one setup but not another, due to a variety of reasons and assay conditions. This is the value Diaclone offers – we provide multiple scFvs and sdAbs to test and choose from in order to find the one that works best for your needs.6
The Medix Biochemica Group offers excellent quality reagents, reliable batch-to-batch consistency and superior customer-focused service.6
Contact our team of experts to discuss your specialty reagent requirements






